- bdttonyjiao@hotmail.com
- +86 +8615081115902
- 9-1-701 TonfuTown Shijiahzuang City Hebei Province China
أغسطس . 28, 2025 05:00 Back to list
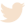
Reliable Sodium EDTA Tube: Sterile Vacuum Blood Collection
Industry Trends and the Critical Role of Blood Collection
The landscape of in-vitro diagnostics (IVD) is continuously evolving, driven by advancements in medical technology, increasing demand for early disease detection, and the global focus on personalized medicine. Central to this evolution is the integrity and quality of biological samples, particularly blood. Accurate diagnosis and effective treatment protocols heavily rely on the initial sample collection process. This is where specialized collection devices, such as the sodium edta tube, play an indispensable role.
The global market for blood collection tubes is projected to grow from an estimated USD 2.5 billion in 2023 to USD 4.1 billion by 2030, at a compound annual growth rate (CAGR) of 7.2%. This growth is fueled by an aging global population, rising prevalence of chronic diseases, and increasing laboratory automation. As laboratories strive for higher throughput and reduced manual errors, the demand for reliable, high-performance blood collection tubes, including various forms of the edta vacuum tube, is escalating. Innovation focuses on enhanced anticoagulant coatings, improved vacuum consistency, and materials compatible with a wider range of diagnostic platforms, ensuring optimal sample preservation for a broad spectrum of tests, from routine hematology to advanced molecular diagnostics.
Technical Specifications and Manufacturing Process Flow
The manufacturing of a high-quality edta tube is a precise, multi-stage process designed to ensure sterility, accuracy, and optimal sample preservation. Understanding this process highlights the commitment to quality and technical excellence required for diagnostic consumables.
Detailed Manufacturing Process Flow for Sodium EDTA Tubes
- Material Preparation (Tube Body): High-grade medical-grade polyethylene terephthalate (PET) or borosilicate glass is selected. PET tubes offer breakage resistance and lighter weight, while glass tubes provide superior gas barrier properties. Materials conform to USP Class VI biological reactivity tests.
- Tube Forming & Molding: For PET tubes, injection molding techniques are used to form the tube body, ensuring precise dimensions and wall thickness. For glass tubes, specialized glass blowing and cutting processes are employed. All processes are conducted in controlled environments to prevent contamination.
- Anticoagulant Dispensing: A precise volume of K2EDTA or K3EDTA solution is dispensed into each tube. The solution concentration and volume are critical for the correct blood-to-anticoagulant ratio, typically 1.8 mg of K2EDTA per 1 mL of blood for a 1:100 ratio. This step is performed by automated, highly accurate dispensing systems.
- Drying & Coating: The dispensed EDTA solution is then dried, usually through a vacuum drying or spray-drying process, to form a thin, uniform coating on the inner surface of the tube. This ensures rapid dissolution upon blood contact and prevents dilution artifacts. Uniformity is paramount for effective coagulation inhibition.
- Stopper Insertion & Vacuum Creation: Medical-grade bromobutyl rubber stoppers are mechanically inserted. During this process, a precise vacuum is created inside the tube, which is essential for drawing a predetermined volume of blood upon venipuncture. Vacuum levels are meticulously controlled and tested.
- Labeling & Packaging: Tubes are labeled with critical information, including lot number, expiration date, and volume. They are then packaged in sterile, easy-to-open trays or racks, typically in quantities suitable for laboratory use.
- Sterilization: The packaged tubes undergo terminal sterilization, most commonly through gamma irradiation or electron beam (E-beam) sterilization, ensuring a sterility assurance level (SAL) of 10^-6, compliant with ISO 11137.
- Quality Control & Testing: Throughout the entire process, rigorous quality control checks are performed. This includes visual inspection, dimension checks, vacuum retention tests, sterility tests, and anticoagulant efficacy tests to confirm adherence to ISO 6710, CLSI GP41, and specific customer requirements.
Product Specifications: EDTANa2 Tube
Our EDTANa2 Tube, designed for optimal performance in hematology, features robust specifications:
| Parameter | Specification | Standard Compliance |
|---|---|---|
| Tube Material | Medical Grade PET (Polyethylene Terephthalate) | USP Class VI |
| Anticoagulant Type | Disodium Ethylenediaminetetraacetic Acid (EDTANa2) | CLSI GP41-A6 |
| Anticoagulant Concentration | 1.8 mg EDTA per mL of blood (typical) | ISO 6710 Annex B |
| Draw Volume Range | 2.0 mL - 10.0 mL (various options) | ISO 6710 (Volume tolerance ±10%) |
| Tube Dimensions (e.g., for 5mL) | 13mm x 75mm, 13mm x 100mm, 16mm x 100mm | N/A (Standardized dimensions) |
| Closure Type | Rubber Stopper (Bromobutyl) | Latex-free |
| Cap Color | Lavender / Purple | ISO 6710, CLSI GP41-A6 |
| Sterilization Method | Gamma Irradiation or E-Beam | ISO 11137 (SAL 10^-6) |
| Shelf Life | 18-24 Months from Manufacturing Date | Validated Stability Data |
These specifications ensure that each sodium edta tube delivered is a product of stringent quality control and advanced manufacturing techniques, providing consistent performance for critical diagnostic procedures.
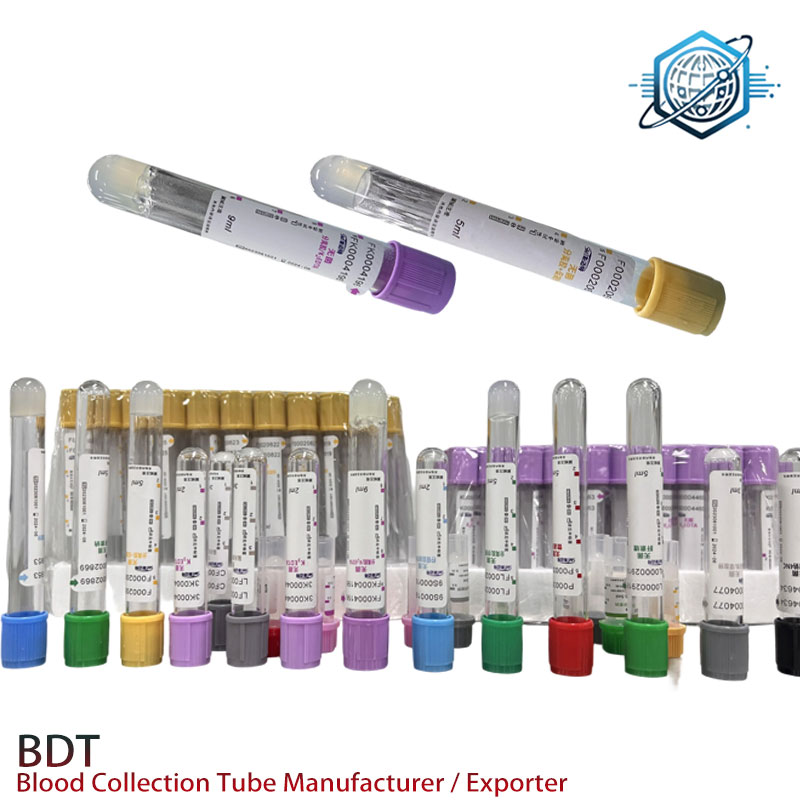
Application Scenarios and Technical Advantages
The specific chemical properties of EDTA make it an ideal anticoagulant for a multitude of diagnostic applications. Its chelation of calcium ions effectively prevents blood coagulation without altering cellular morphology, a critical factor for accurate laboratory analysis.
Key Application Scenarios:
- Hematology: The primary application for sodium edta tubes is in complete blood count (CBC) and differential blood cell counts. EDTA preserves cellular components (red blood cells, white blood cells, platelets) without causing significant morphological changes for up to 24 hours at room temperature, allowing for accurate automated and manual analysis. Other tests include erythrocyte sedimentation rate (ESR), reticulocyte counts, and hemoglobin A1c.
- Blood Banking: EDTA tubes are crucial for various pre-transfusion tests, including blood grouping, Rh typing, and cross-matching, due to their ability to prevent coagulation while maintaining cellular integrity. They are also used for antibody screening and molecular typing of blood groups.
- Molecular Diagnostics: For tests requiring DNA or RNA extraction from whole blood, EDTA is the preferred anticoagulant. It inhibits nuclease activity and preserves nucleic acid integrity for applications like PCR, DNA sequencing, and genetic testing.
- Immunology and Virology: Used for viral load testing (e.g., HIV, HBV, HCV), flow cytometry, and other immunological assays where cellular components need to be preserved in their native state.
Technical Advantages:
- Optimal Cellular Preservation: EDTA's mechanism of action effectively prevents clot formation without causing significant erythrocyte shrinkage or swelling, platelet aggregation, or leukocyte degradation, which is crucial for accurate cell counts and morphology assessments.
- Compatibility with Automation: Our edta vacuum tubes are designed for seamless integration with automated hematology analyzers, ensuring consistent sample quality and reducing the need for manual intervention, thereby improving laboratory efficiency and throughput. The precision of the vacuum draw volume is critical here.
- Reliable Vacuum Performance: The manufacturing process guarantees a stable vacuum, drawing the correct volume of blood consistently. This precision is vital for maintaining the anticoagulant-to-blood ratio, which directly impacts the accuracy of diagnostic results.
- Reduced Risk of Sample Contamination: With sterile manufacturing processes and robust stopper designs, the risk of external contamination is minimized, protecting the integrity of the collected sample and ensuring patient safety.
- Extended Sample Stability: Properly collected in an edta tube, blood samples can maintain stability for specific parameters (e.g., CBC) for several hours at room temperature and longer under refrigeration, allowing for flexibility in laboratory processing.
Vendor Comparison and Customized Solutions
Choosing the right vendor for blood collection tubes is a strategic decision for any healthcare institution or diagnostic laboratory. Factors such as product quality, consistency, regulatory compliance, and support services are paramount.
Vendor Comparison: Key Differentiators
| Feature/Service | BDTheatingpads (EDTANa2 Tube) | Competitor X (General Market) | Competitor Y (Budget Option) |
|---|---|---|---|
| Manufacturing Standards | ISO 13485, CE, FDA Registered (if applicable) | ISO 13485, CE | Basic Quality Control |
| Anticoagulant Quality & Purity | High Purity (Ph. Eur./USP grade) for optimal chelation | Standard Pharmaceutical Grade | Industrial Grade |
| Vacuum Consistency | ±5% variation across batches (automated testing) | ±10% variation (batch testing) | Variable, less rigorous testing |
| Customization Options | Extensive (volume, concentration, label, packaging) | Limited (volume, label) | Minimal to none |
| Technical Support | Dedicated technical team, prompt response | Standard customer service | Basic inquiry support |
| Traceability & Batch Control | Full end-to-end traceability per ISO 13485 | Batch-level traceability | Limited traceability |
Customized Solutions for Specific Needs
Recognizing that diverse laboratory environments have unique requirements, BDTheatingpads offers a comprehensive suite of customization options for our edta tubes:
- Variable Draw Volumes: From micro-collection tubes (0.5 mL) to larger volume tubes (up to 10 mL), precisely calibrated to specific diagnostic needs.
- Anticoagulant Concentrations: While standard concentrations are common, specific research or diagnostic protocols may require modified EDTA concentrations (e.g., lower concentrations for certain molecular assays).
- Custom Labeling & Barcoding: Integration of laboratory-specific identifiers, logos, or 2D barcodes to streamline sample tracking and reduce manual data entry errors.
- Alternative Tube Materials: While PET is standard, specialized applications might benefit from glass tubes for long-term storage or specific gas barrier requirements.
- Packaging Configurations: Custom tray counts, bulk packaging, or specialized secondary packaging to fit automated pre-analytical systems or specific storage conditions.
- Special Additives: For certain research applications, additional components can be incorporated upon request, after thorough validation.
Our technical team collaborates closely with clients to develop and validate these customized solutions, ensuring that the tailored vacuum tube edta meets all performance and regulatory benchmarks.

Application Case Studies
Real-world application demonstrates the tangible benefits of high-quality sodium edta tubes in various critical settings.
Case Study 1: Large Academic Medical Center – Enhanced Hematology Workflow
A prominent academic medical center was experiencing inconsistent blood draw volumes and occasional micro-clot formation, leading to repeat collections and delayed diagnostic turnaround times. Upon transitioning to our EDTANa2 Tubes, the lab reported a significant improvement. The precise vacuum control and uniform EDTA coating ensured consistent blood-to-anticoagulant ratios, virtually eliminating micro-clots and reducing redraw rates by 18%. This directly improved workflow efficiency, minimized patient discomfort from repeat venipuncture, and accelerated the delivery of critical CBC results for patient care. The compatibility with their existing automated analyzers was seamless, requiring no recalibration.
Case Study 2: National Blood Bank – Optimizing Donor Sample Integrity
A national blood bank, responsible for screening thousands of donor samples daily for infectious diseases and blood typing, sought to enhance the stability and integrity of their samples during transport and storage. Their previous tubes sometimes showed signs of cellular degradation or slight hemolysis after extended transit. By implementing our EDTANa2 Tubes, specifically designed with advanced PET material for better barrier properties and optimized anticoagulant drying, they observed superior preservation of cellular components and nucleic acids. This ensured more reliable results for molecular assays and serological tests, ultimately safeguarding the blood supply and improving donor management.
Case Study 3: Pharmaceutical Research Laboratory – Consistent Sample Quality for Clinical Trials
A pharmaceutical company conducting multi-center clinical trials faced challenges in standardizing blood sample collection across diverse geographical sites. Variability in tube quality from local suppliers led to discrepancies in hematological parameters, impacting study data. Adopting a standardized procurement of our EDTANa2 Tubes across all trial sites provided unparalleled consistency in sample quality. This reduced inter-site variability in hematology results, strengthened the reliability of their clinical trial data, and streamlined regulatory submissions, showcasing the critical importance of a consistent, high-quality vacuum tube edta in research.
Trustworthiness, Support, and Quality Assurance
At BDTheatingpads, our commitment extends beyond product delivery to fostering long-term trust and ensuring operational excellence for our clients.
Frequently Asked Questions (FAQ)
- Q: What is the recommended storage condition for EDTANa2 Tubes?
- A: Our sodium edta tubes should be stored at room temperature (4-25°C), away from direct sunlight or extreme temperature fluctuations. Do not refrigerate or freeze, as this can affect vacuum stability and tube material integrity.
- Q: Are your EDTA tubes compatible with all automated hematology analyzers?
- A: Yes, our tubes are designed to be compatible with a wide range of commercially available automated hematology analyzers. However, we always recommend verifying compatibility with your specific instrument manufacturer's guidelines and performing internal validation.
- Q: What is the shelf life of your EDTANa2 Tubes?
- A: Our EDTANa2 Tubes have a typical shelf life of 18-24 months from the date of manufacture, depending on the specific product configuration and storage conditions. The exact expiration date is printed on the label of each tube and packaging.
- Q: Can I request a sample of your EDTANa2 Tubes for evaluation?
- A: Absolutely. We encourage prospective clients to evaluate our products. Please contact our sales team to discuss sample requests and evaluation protocols.
Lead Time and Fulfillment Details
We maintain efficient production schedules and robust inventory management to ensure timely delivery. Standard lead times for stock items typically range from 2-4 weeks. For customized orders, lead times will be confirmed based on complexity and volume, usually between 6-10 weeks after design approval. Our logistics team works with global shipping partners to ensure secure and efficient delivery worldwide, with options for expedited shipping if required.
Warranty Commitments
BDTheatingpads stands behind the quality of its EDTANa2 Tubes with a comprehensive warranty against defects in materials and workmanship. Our products are guaranteed to meet the published specifications and industry standards (ISO 6710, CLSI GP41) for their stated shelf life under proper storage and usage conditions. Any product failing to meet these standards will be promptly replaced or credited, subject to our quality assurance investigation.
Customer Support and Technical Assistance
Our dedicated customer support team and technical specialists are available to assist with product inquiries, application guidance, and troubleshooting. We offer multi-channel support including email, phone, and online resources. For complex technical challenges, our R&D team is available for in-depth consultation, ensuring that you receive expert advice and solutions for any specific needs related to our edta vacuum tube products. We are committed to partnership and continuous improvement.
Certifications and Authoritative References
Our commitment to excellence is underpinned by adherence to stringent international quality and regulatory standards.
- ISO 13485 Certified: Our manufacturing facilities operate under an ISO 13485 certified Quality Management System, ensuring consistent quality and regulatory compliance for medical devices.
- CE Mark: Our EDTANa2 Tubes comply with the essential requirements of the European Medical Device Regulation (MDR), qualifying them for distribution within the European Economic Area.
- FDA Registered: (If applicable) Our products are registered with the U.S. Food and Drug Administration, demonstrating compliance with U.S. regulatory standards for medical devices.
- CLSI & ISO Standards: All product development and testing adhere to relevant Clinical and Laboratory Standards Institute (CLSI) guidelines (e.g., GP41 for blood collection tubes) and International Organization for Standardization (ISO) standards (e.g., ISO 6710 for single-use container111s for human venous blood specimen collection).
- Years of Service: With X years of specialized experience in medical device manufacturing, we have built a reputation for reliability and innovation.
- Partner Clients: Our extensive client portfolio includes leading hospitals, research institutions, and diagnostic laboratories globally, testament to our proven track record.
References:
- Clinical and Laboratory Standards Institute (CLSI). (2017). Procedures for the Collection of Diagnostic Blood Specimens by Venipuncture; Approved Standard—Seventh Edition. CLSI document GP41. Wayne, PA: Clinical and Laboratory Standards Institute.
- International Organization for Standardization. (2017). ISO 6710:2017 - Single-use container111s for human venous blood specimen collection.
- World Health Organization (WHO). (2010). WHO guidelines on drawing blood: Best practices in phlebotomy. Geneva: World Health Organization.
This is the first article
-
Premium Heating Pads for Pain Relief & Comfort
NewsAug.27,2025 -
Cozy Home Foot Warmer: Ultimate Comfort & Relief for Cold Feet
NewsAug.26,2025 -
Lithium Heparin Capillary Tubes for Blood Collection - Precise & Safe
NewsAug.21,2025 -
Accurate Sodium Fluoride EDTA Tubes for Glucose
NewsAug.19,2025 -
Electric Hot Pad: Soothe Pain & Stay Warm with Targeted Heat
NewsAug.18,2025