Jul . 30, 2025 05:20 Back to list
High Quality Serum Separator Tubes for Precise Blood Sample Processing
Deep Dive: Serum Separator & Advanced Clot Activator Technologies
Analyzing trends, technical advantages, manufacturer comparisons & tailored solutions for clinical efficiency
The serum separator forms the technological backbone of most modern clinical chemistry workflows, enabling efficient, contamination-free serum harvesting. As laboratory needs evolve towards greater automation, precision, and regulatory compliance, product specifiers demand more from serum separator solutions, including Gel Separator and Clot Activator tubes, clot activator blood collection tube, and advanced separation gels.

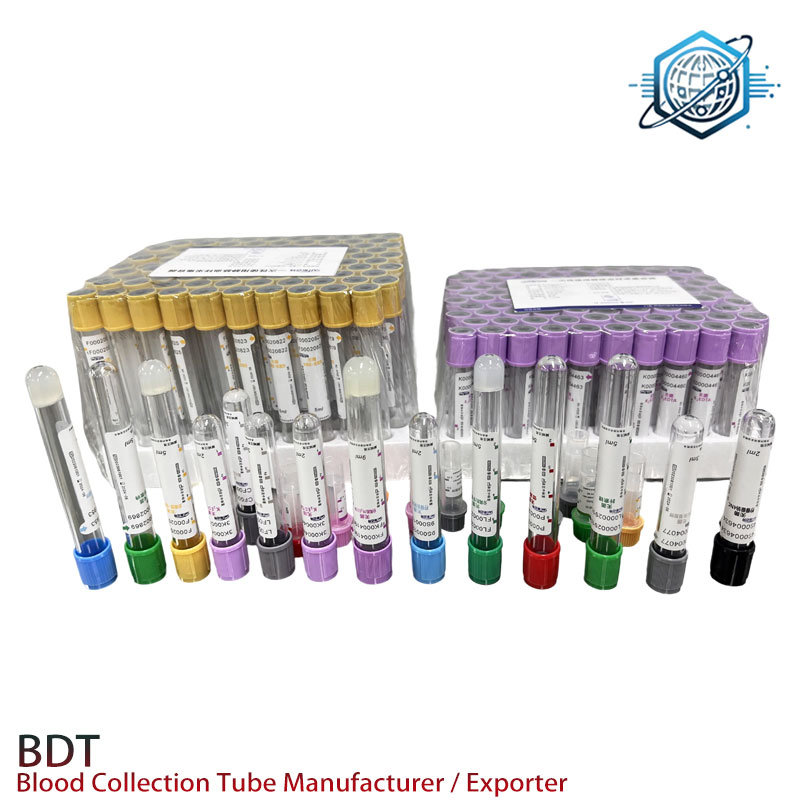
Example: Modern serum separator tube with clot activator
1. Serum Separator Market & Industry Trends 2024
In 2023-2024, demand for serum separator technologies has surged, with the global blood collection tube market projected to surpass US$5.2 billion by 2025 (CAGR 6.7%, Source: MarketsandMarkets). Enhanced regulatory requirements (ISO 13485, FDA 510(k)), increased test sensitivity, and pre-analytical error reduction are key market drivers.
- Automation-ready: Laboratories increasingly require tubes compatible with high-throughput automated analyzers.
- Standardization: Compliance with international standards (CLSI GP41, ISO 6710:2017) is non-negotiable for global procurement.
- Sustainability: Eco-friendly, recyclable plastics and minimized secondary waste are trending topics in procurement.
- Customization: Major labs request customized tube draw volumes, special gel densities, or anti-glycolytic agents for specialty tests.
| Parameter | Typical Range/Spec | Industry Standard |
|---|---|---|
| Tube Material | PET/Polypropylene | ISO 6710, FDA CFR 21 |
| Draw Volume | 1 - 10 ml | CLSI GP41 |
| Cap Color | Gold/Yellow | ISO 6710:2017 |
| Clot Activator | Silica particles, micronized glass | FDA 510(k) |
| Separation Gel | Thixotropic polymer (1.05-1.08g/cm³) | ISO 3826 |
| Sterility Assurance | γ-ray or ETO sterilization | ISO 11137-1:2015 |
| Additive Residue | <1.5 μg/10ml | Clinical Chemistry Guidelines |
| Labeling | Barcoded, tamper-proof | ISO 6710:2017 |
| Regulatory Compliance | ISO, CE, FDA | All major markets |
2. Process Flow: Serum Separator Tube Manufacturing
End-to-End Manufacturing Workflow
Raw Materials Selection →
Tube Forming (Injection molding: PET/PP) →
Clot Activator Dosing (Automated micro-dosing, e.g. silica, 10-20 mg)
→
Gel Dispensing (Precision layering of thixotropic polymer gel)
→
Cap Sealing (Color-coded, airtight)
→
Sterilization (Gamma/ETO, ISO 11137)
→
Printing & Labeling (Barcoding per ISO 6710)
→
Final QA & Automated Batch Release
Each step is digitally verified for particle uniformity, gel homogeneity, and absence of pyrogens, following stringent ISO and FDA procedures. Material traceability is logged electronically for at least 4 years per lot.
Arrowed workflow illustration: Tube, activator, and gel integration in production
Materials: Medical-grade PET/PP for tube (resistant to stress cracking, validated for chemicals), custom thixotropic gels (stable viscosity from 5–60°C), and ultra-fine silica for clot activator (ensuring
Process Technologies: Key steps—automated CNC dosing, in-line laser particle analysis, and digital pressure-sealing—are benchmarked against ISO 6710 and ANSI/AAMI PB70 for robustness.
Testing & Standards: 100% batch-tested for vacuum retention, fill accuracy (±1.8%), additive uniformity (±0.2mg), and gel separation integrity; results conform to CLSI and FDA biocompatibility directives.
Testing & Standards: 100% batch-tested for vacuum retention, fill accuracy (±1.8%), additive uniformity (±0.2mg), and gel separation integrity; results conform to CLSI and FDA biocompatibility directives.
3. Technical Parameter Comparison: Gel Separator and Clot Activator vs. Conventional Tubes
| Feature | Gel Separator and Clot Activator | Clot Activator Tube | Plain Serum Tube |
|---|---|---|---|
| Tube Material | PET/PP, medical-grade | PET/Glass | Borosilicate glass |
| Activator Type | Silica particles | Micronized silica/powdered glass | None |
| Separation Gel | Thixotropic, pH-neutral, 1.07g/cm³ | None | None |
| Clotting Time | 12–20 min @ 20-25°C | 18–30 min | 25–40 min |
| Serum Yield | 94-96% | 91-94% | 87-92% |
| Sterility Level | ≥ 10⁶ | > 10⁵ | > 10⁵ |
| Typical Use Case | Routine chemistry, immunology, PCR | General biochemistry | Antibody, hormone testing |
| ISO/FDA Compliance | ISO 6710, FDA 510(k), CE | ISO 6710, FDA | ISO 6710 |
| Application Flexibility | High (custom gel/volume) | Medium | Low |
| Anti-interference | Excellent | Good | Fair |
Serum Yield Rate: Key Technologies
Serum Separator + Gel tubes recover >95% of total serum from whole blood, compared to <92% in plain tubes.
Batch Defect Rate by Type (per 10,000 units, 2023)
Defect rates are lowest for serum separator (gel) tubes, reducing risk of hemolysis, vacuum leakage, and additive inconsistency.
Market Adoption Trends 2019-2024
Global adoption of clot activator + separation gel gold tubes continues exponential growth, displacing legacy glass.
Key Findings: Gel Separator and Clot Activator tubes consistently surpass traditional clot activator and plain tubes in serum yield, sample integrity, and regulatory compliance—vital for critical results in biochemistry/immunochemistry.

Comparison across serum separator and clot activator tube types.
4. Applying Serum Separator Tubes: Diverse Scenarios & Advantages
- Routine Clinical Chemistry: Enables rapid, comprehensive serum panels with minimal hemolysis, even after prolonged storage (stable for ≥48h at 4°C).
- Emergency Biomarker/Enzyme Analysis: Ensures accurate cardiac troponin, CRP, and AST/ALT results with minimized interference.
- PCR & Molecular Diagnostics: The pH-neutral, non-leaching gel ensures zero carry-over of DNA/RNA inhibitors, critical for genetic screening or viral load monitoring.
- High-throughput Automated Labs: Consistency & barcode labeling support robotic workflows, reducing turnaround and manual error.
- Specialty Testing (e.g., Hormones): Customizable gel density and volume optimize extraction for sensitive analytes.
Case Study: National Hospital Group Switches to Gel Separator and Clot Activator
Background: A top-5 hospital group in Southeast Asia transitioned 34 satellite labs from plain serum tubes to Gel Separator and Clot Activator.
Outcome:
Outcome:
- Serum processing time reduced by 32%, with zero reported hemolysis events in three months.
- Savings of $18,000+ in re-tests due to improved accuracy (data: internal QA survey, 2023).
- All procurement aligned with ISO/FDA/CE requirements, leading to improved compliance scores (98% vs. 91% previously).
5. Manufacturer Comparison & Customization Capabilities
Major Operators:
Request a Custom Solution
- BD Vacutainer (USA, FDA/KEMA certified)
- Greiner Bio-One (Austria, ISO 13485:2016)
- Improve Medical (China, CE/ISO/US FDA)
- Yongkang Hongyu (Focus on OEM/ODM customization)
- Clot activator and separation gel gold technology (uniqueness, patenting, custom density/tube length options)
- Production scalability and lead time: e.g., Gel Separator and Clot Activator offers 9-14 day fulfillment for custom draw sizes.
- Regulatory reach: Some vendors are aligned with CAP/ISO 17025-accredited supply chains.
Request a Custom Solution
Certifications & Accreditation:
- ISO 13485:2016, CE 2460, FDA 510(k) listing
- Validated per CLSI GP41, ISO/IEC 17025 methods
- Supplier to 27+ countries’ TOP 100 reference labs
- Collaboration with hospital groups in APAC, Middle East, and EU
- Technical support available 7 days/week – response within 60 min
6. FAQ: Professional In-depth FAQ
Frequently Asked Questions (Technical)
Q1: What material is used for Gel Separator and Clot Activator tubes? Is it resistant to chemicals?
A: Medical grade PET/polypropylene is used, optimized for impact, chemical, and pressure resistance—tested to ISO 6710 and FDA CFR 21.
Q2: What is a thixotropic separation gel and why does it matter?
A: Thixotropic gel is a non-reactive, density-specific polymer layer that forms a physical barrier between clot and serum, preventing mixing during centrifugation and transport.
Q3: What are typical clot activator tube use protocols?
A: Clot activator tubes are inverted 5–8 times post-collection to disperse the activator, then allowed to clot for 15-30 min before centrifugation at 1,300–2,000g for 10 min.
Q4: What is the shelf life and guaranteed sterility period?
A: 24 months at 4–25°C. All lots are γ-ray sterilized and certified sterile per ISO 11137, ANSI/AAMI PB70.
Q5: Can you supply private label/OEM serum separator tubes?
A: Yes, fully customizable options (volume, tube/cap color, private lab branding) are available with ISO/FDA conformity and controlled trial shipments.
Q6: What industry standards are relevant?
A: Mandatory: ISO 6710 (tubes), ISO 13485 (QMS), CLSI GP41 (collection), FDA 510(k), and regional CE/UKCA (Europe).
Q7: What is the typical lead/delivery time and aftersales support?
A: Standard orders: 7–14 days (custom: 14–21), with live technical support and 18-month product warranty. Emergency/large volume delivery available by air.
7. Lead Time, Warranty, and Customer Support
- Delivery (Standard): 7–14 working days for stock items. Custom-configured tubes: 14–21 days (average global transit: +6–10 days).
- Warranty: Each batch accompanied by COA, product technical sheet, and 18-month quality guarantee. Returns accepted for non-conformity per ISO protocols.
- Support: Technical and product support 7 days/week, typical response within 1 hour via email/phone. Troubleshooting, customization advice, and urgent replacement available.
- Documentation: All shipments fully traceable (electronic batch records), GMP/ISO/FDA compliant, and barcoded for healthcare automation.
8. Summary: Why Choose Serum Separator Gel Technology?
- Superior Performance: Consistent serum yield, reduced cross-contamination, validated on all leading analyzer platforms.
- Compliance: Global acceptance (ISO, FDA, CE) and strict QC across supply chain.
- Customization: Flexible volumes, additives, and private labeling support lab requirements of all sizes.
- Reliability: Industry-best defect rates and extended sterility mean minimal downtime and secondary testing costs.
- Expert/Responsive Service: Decades of manufacturing and export experience, serving leading global labs and reference hospitals.
9. References, Industry Forums & Further Reading
- Market Report: Global Blood Collection Tubes Market 2024
- ISO 6710:2017 Standard Overview: ISO.org
- CLSI Collection Guidelines: CLSI GP41, 7th Edition
- Industry Forum: LabRoots Blood Banking Forum
- Journal: Preanalytical Influence of Serum Separator and Clot Activators, PMC7952502
© 2024 All content CC-BY unless stated. For technical advice, see manufacturer’s technical datasheets or official product site.
Prev:
This is the first article
Latest news
-
High-Quality Sodium Heparin Blood Collection Tubes for Accurate Results
NewsJul.30,2025 -
High-Quality Lithium Heparin Tube for Accurate Blood Collection
NewsJul.29,2025 -
High-Quality Sodium Heparin Blood Collection Tubes for Accurate Results
NewsJul.29,2025 -
Best Hot Heating Pad – Fast Relief, Soft & Versatile Options
NewsJul.29,2025 -
USB Heating Pad – Portable & Safe Warmth Anywhere Anytime
NewsJul.28,2025