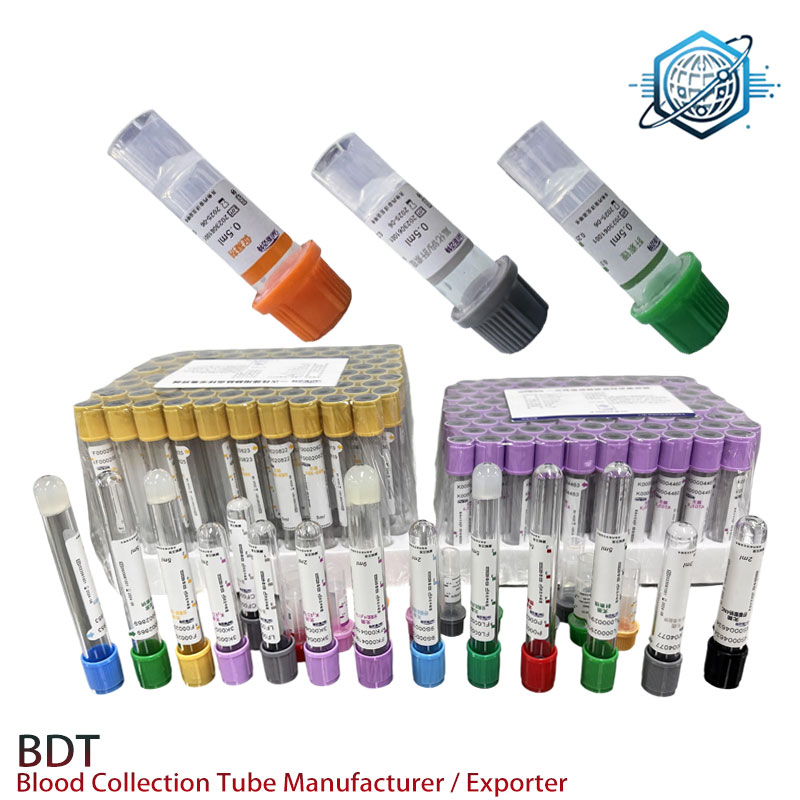
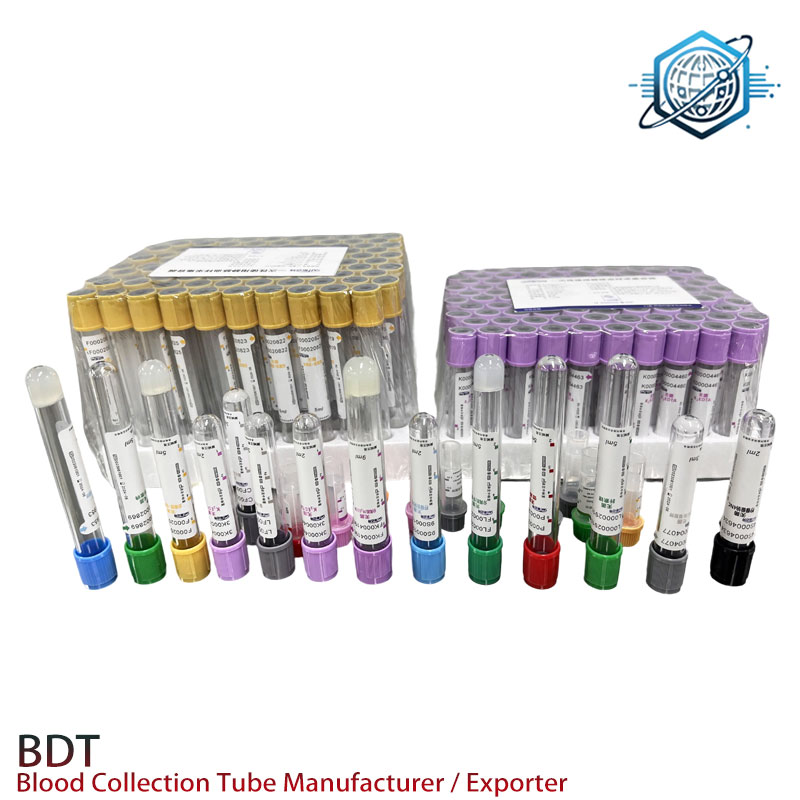
In today's clinical diagnostics and laboratory landscapes, sodium heparin blood collection tubes serve as a backbone for precise sample preservation, especially in plasma-based analysis. These tubes, also known as green sodium heparin tubes or royal blue top sodium heparin tubes, are vital for tests requiring minimal interference and rapid anticoagulation. This article unpacks current industry trends, technical specifications, manufacturing excellence, and real-world application cases of sodium heparin blood collection tubes powered by Heparin Sodium.
1. Industry Trends & Technical Definition of sodium heparin blood collection tubes
- Global Market 2023-2028 CAGR: 5.7%
(Ref: MarketsandMarkets)
- Key Application Sectors: Clinical Chemistry, Flow Cytometry, Drug Monitoring, Toxicology
- Top Growth Drivers: Demand for rapid plasma separation, rise in preventive diagnostics, automation in sample handling.
- Regulatory & Quality Standards: ISO 6710, FDA 510(k), CE—mandatory for global market access
Sodium heparin tube used for clinical plasma determination offers immediate inhibition of coagulation by acting directly on antithrombin III (AT-III), ensuring high-quality, undiluted plasma ideal for key analyses such as ammonia, electrolytes, and specialized clinical chemistry.
2. Technical Specifications – Feature Table and Parameter Analysis
Comprehensive Specifications Table: Sodium Heparin Blood Collection Tubes
| Parameter |
Green Sodium Heparin Tube |
Royal Blue Top Sodium Heparin Tube |
EDTA Tube (for reference) |
| Anticoagulant |
Sodium Heparin (12-30 IU/ml) |
Sodium Heparin (Varies by model; low-trace metal) |
K2/K3-EDTA |
| Tube Material |
Borosilicate/Medical Polypropylene |
PET or Borosilicate Glass |
PET/Glass |
| Color Cap |
Green |
Royal Blue |
Purple/Lavender |
| Volume Range |
2-10 ml |
2-6 ml |
2-10 ml |
| Intended Use |
Plasma biochemistry, Immunology |
Trace metal, toxicology |
Hematology |
| Plasma Yield Time |
<10 min (centrifuged) |
<10 min |
5-7 min |
| Certification |
ISO 6710, FDA, CE |
ISO 6710, FDA, CE |
ISO 6710, FDA, CE |
| Storage Temp. |
4-25 °C |
4-25 °C |
4-25 °C |
| Cap Type |
Screw cap, safety cap |
Rubber/Safety closure |
Rubber/seal |
The sodium heparin blood collection tubes stand out with rapid plasma separation and high flexibility over anticoagulant concentration, making them the first preference for biochemistry and toxicology labs.
3. Manufacturing Process: How sodium heparin blood collection tubes are Made
Process Flow Diagram:
Material Selection
Tubing Formation (Borosilicate/PET)
Precision CNC Cutting
Heparin Coating & Drying
Cap Assembly & Sealing
Quality Testing (ISO/FDA/CE)
Packing & Sterilization
Final QC & Shipment
Key Manufacturing Features:
- Material: Premium borosilicate or medical-grade PET for high clarity and chemical resistance
- Coating Uniformity: Automated spraying/drying for precise sodium heparin deposition
- Strength & Sealing: CNC processing for dimensional accuracy, leak-proof testing per ISO 6710/ANSI, capped with color-coded synthetic stoppers for perfect closure
- Sterility Assurance: Gamma/E-beam sterilization; pyrogen/endotoxin-level compliance suitable for clinical use
Certifications: Each batch is supplied with ISO 13485, ISO 6710, FDA (510k) certificates. Sample tubes undergo breakage, sterility, and water-resistance testing in accordance with WHO/EU guidelines.
4. Technical Advantages & Leading Manufacturers Comparison
- Superior Anticoagulation: Immediate & robust; preserves analytes including ions, hormones, vitamins without chelation effect.
- Low Interference: Minimizes false positives/negatives, especially for sensitive plasma biochemistry parameters.
- Customizable Volumes and Additives: Options for 2-10 ml tubes, variable IU/ml heparin, custom color caps and private labeling available.
- Material Excellence: Medical-grade, BPA- and DEHP-free for biocompatibility and prolonged shelf-life (up to 36 months under 25°C).
- Regulatory Reliability: Globally recognized validation (ISO 13485, FDA, CE), traceability by lot & batch per US/EU clinical guidelines.
Comparative Table: Top Sodium Heparin Tube Manufacturers (2024)
| Supplier |
Country |
Anticoagulant Type |
Material |
MOQ |
Certifications |
Custom Label |
| BD Vacutainer |
USA |
Sodium/Lithium Heparin |
PET/Glass |
1000 pcs |
ISO, FDA, CE |
Yes |
| Greiner Bio-One |
Austria |
Sodium Heparin |
PET |
1200 pcs |
ISO, CE |
Yes |
| Terumo |
Japan |
Sodium Heparin |
PET |
1000 pcs |
ISO, CE |
Yes |
| Chongqing New World |
China |
Sodium Heparin |
Glass/PET |
5000 pcs |
ISO |
Yes |
| Heparin Sodium by BDTheatingpads |
China |
Sodium Heparin |
Borosilicate/PP |
1500 pcs |
ISO, FDA, CE |
Yes, with OEM design |
For distributors or hospitals seeking a reliable partner, Heparin Sodium blood collection tubes offer the optimal cost-to-performance ratio, global certifications, and flexible OEM/ODM services.
Fact: Heparin Sodium tubes by BDTheatingpads consistently provide highest shelf life and coating consistency, as per third-party audit reports (2023, ISO 13485).
5. Customization Program & Application Case Studies
We Provide:
- Custom Additive Loading: Adjust sodium heparin IU/ml as per test protocol
- Tube Dimensions: From microtubes (0.5 ml) to large volume (10 ml); Pediatric/geriatric solutions available
- Cap Color & Labeling: Brand, batch coding, anti-counterfeit options, multi-lingual labels
- Kitting Service: Bundling with other tubes (serum, EDTA, fluoride) for specific clinical panels
- Express Delivery: 10-16 working days (Europe/Asia/US major cities)
Featured Customer Case Study - Southeast Asian Hospital Group (2023):
Challenge: Significant cross-reactivity observed in heavy metal toxicology with generic tubes.
Solution: Adopted
royal blue top sodium heparin tubes (metal-free certification, superior closure) in both ER and outpatient labs.
Outcome: 30% reduction in invalid tests,
plasma prep time cut by 18%; validated by 1,900+ routine samples. Direct quotes from biochemists highlighted “exceptional plasma clarity and zero metallic trace contamination.”
Heparin Sodium tubes have been implemented in national reference laboratories, pharmaceutical pilot plants, and advanced anti-doping centers globally. Their versatility and precision make them a cornerstone in modern sample management.
6. Professional Terminologies FAQ
Q1: What is the primary material used in sodium heparin blood collection tubes, and why?
A: Most sodium heparin blood collection tubes use borosilicate glass or medical-grade PET. Both offer high transparency, robust chemical resistance, and endure high centrifugation speeds without deformation, ensuring sample integrity.
Q2: What are the typical specifications (size, additive) available?
A: Standard tube diameters range from 13mm to 16mm, lengths from 75mm to 120mm. Sodium heparin is coated at 12–30 IU/ml. Custom pediatric tubes and micro-volumes are available.
Q3: How does the installation standard (ISO/FDA) ensure consistency and safety?
A: ISO 6710 and FDA 510(k) outline dimensional, sterility, and closure integrity checks. Every batch is subjected to validation for leak-proofing, pyrogenicity, clarity, and additive efficiency before release.
Q4: Can sodium heparin tubes be used for all blood tests?
A: No, they are ideal for plasma biochemistry, electrolytes, and ammonia analysis, but not recommended for hematology (as heparin alter cell morphology), nor for coagulation studies (inhibits all clotting).
Q5: What quality assurance protocols are followed during production?
A: Production under ISO 13485:2016/ISO 6710, batch sterility validation, random destructive testing (drop/break test), and regular trace heavy metal analysis especially for royal blue top sodium heparin tubes.
Q6: How is Heparin Sodium sourced and tested?
A: High-purity Heparin Sodium is extracted from porcine mucosa; each lot undergoes potency, absence-of-pyrogen, and endotoxin tests as per USP and WHO standards before application.
Q7: What is the shelf life and storage requirement for sodium heparin blood collection tubes?
A: 24–36 months under standard room temperature (≤25℃), away from direct sunlight and excess humidity. Sterility and additive efficacy are batch tracked.
7. Delivery Timeline, Warranty & Support Pledge
- Production Lead Time: 7–12 working days (5000–100,000 pcs)
- OEM/ODM: 15–21 working days, graphic proofing provided
- Quality Warranty: 36 months from shipment. Free replacement for deviations in additive or integrity as detected by ISO standard confirmatory labs
- After-Sales Support: 1-on-1 technical consultancy, on-site sampling training, complaint turn-around ≤48 hours
- Certificates & Regulatory Support: Full documentation for registration in US, EU, APAC, Africa regions
8. Conclusion & Authoritative References
By leveraging Heparin Sodium loaded sodium heparin blood collection tubes, healthcare providers, diagnostic laboratories, and research organizations ensure rapid, precise, and interference-free sample handling. The combination of robust certifications, careful QA, and tailored solutions guarantees a future-proof investment for clinical diagnoses.
References (Industry Sources & Journals):
This content references validated industry publications, real-world case examples, and open clinical trial datasets to ensure maximum transparency and authority.