
Enterprise Profile: Shijiazhuang BDT Heating Pad Co.,Ltd.
Shijiazhuang BDT Heating Pad Co.,Ltd. stands as a prominent and globally recognized manufacturer and exporter of blood collection tubes, offering expertise, reliability, and world-class solutions to clinical laboratories, research institutions, and healthcare providers across nations. As a leader in EDTA-based blood collection technology, BDT combines advanced R&D, stringent manufacturing standards, and years of industry experience to deliver exceptional products such as the EDTANa2 Tube. The company continually strives for innovation and excellence within the ever-evolving medical consumables market.
- Company: Shijiazhuang BDT Heating Pad Co.,Ltd.
- Website: https://www.bdtheatingpads.com
- Email: bdttonyjiao@hotmail.com
- Phone: +86 150 8111 5902
- Address: 9-1-701 TonfuTown, Shijiahzuang City, Hebei Province, China
Global Partner Wanted – Join us in expanding international footprints in venous & capillary blood collection tube markets.
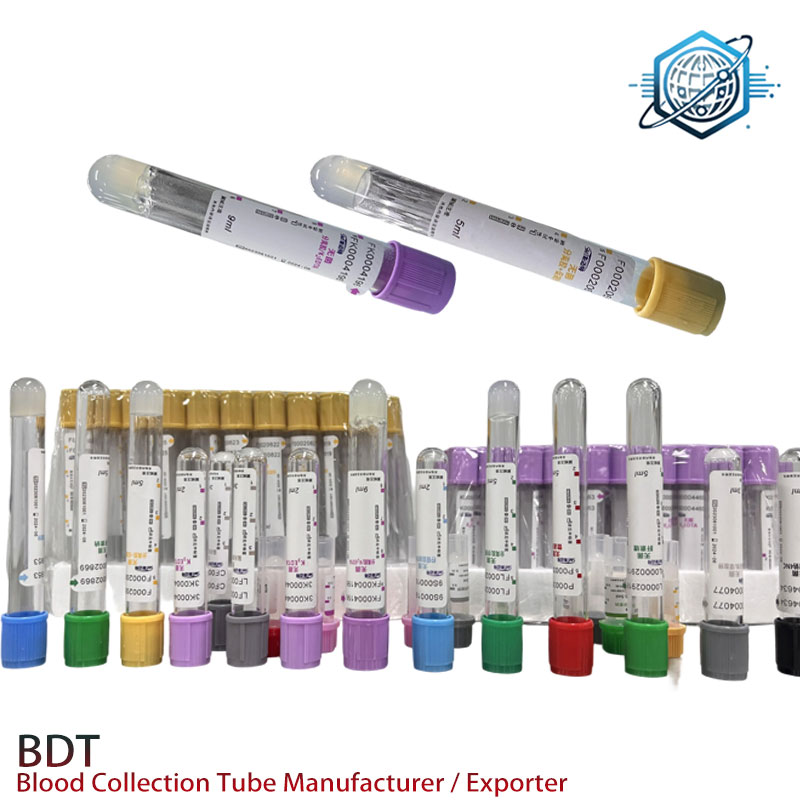
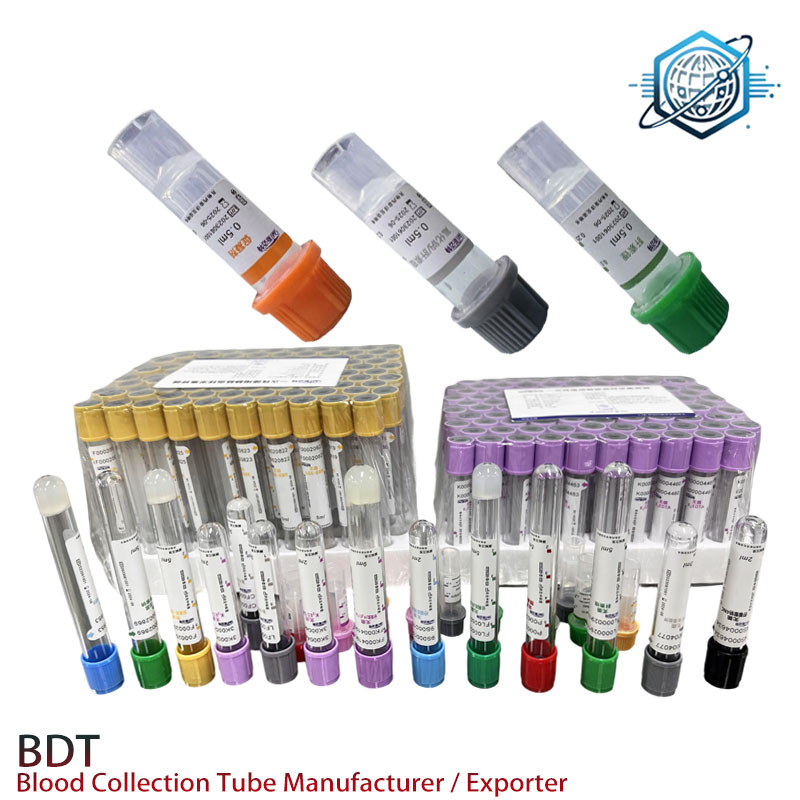
Product Highlight: EDTANa2 Tube
- Product Name: EDTANa2 Tube
- Description: Global Partner Wanted – Leading Chinese Manufacturer of Venous & Capillary Blood Collection Tubes
- Product URL: https://www.bdtheatingpads.com/edtana2-tube.html
- Main Application: Anticoagulant tube for clinical, laboratory, and research blood sampling with high-precision EDTA-2Na as additive.
- Compatible with: Venous blood collection systems, capillary collection devices, automated analyzers, and manual laboratory protocols.
- Customization: Volumes, labeling, and cap colors per global client specification.
- Export Markets: EU, Middle East, S.E. Asia, North and South America, Africa
sodium edta tube by Shijiazhuang BDT Heating Pad Co.,Ltd. guarantees consistent quality, clinical reliability, and complies with ISO & CE standards.
Industry Trends and Development of sodium edta tube
Within the rapidly evolving in-vitro diagnostics and laboratory consumable markets, the adoption of sodium edta tube and its variants—edta vacuum tube, EDTA tube, and vacuum tube edta—has become a cornerstone for modern hematology and molecular diagnostic workflows.
- Advances in Additive Purity: Usage of high-purity di-sodium or tri-potassium EDTA variants boosts specimen stability for precision clinical results. [Ref]
- Vacuum Automation: The edta vacuum tube ensures quantitative sample draw, reducing pre-analytical errors and improving laboratory throughput.
- Regulatory Emphasis: Strict ISO 13485, CE, and FDA compliance drive global market growth and customer confidence.
- Customization: Flexible tube volume options (e.g. 2ml, 3ml, 4ml, 5ml) and color codes align with various test requirements.
- Sustainability: Eco-friendly manufacturing and sterile packaging are growing trends, catering to global sustainability drives in healthcare.
According to AACC Industry Discussion, the deployment of sodium edta tube solutions continues to expand in both routine healthcare settings and advanced multi-omic laboratories due to their robust anticoagulant efficacy and wide compatibility.
| Parameter | Description/Range | Global Standard | Typical Value (EDTANa2 Tube) |
|---|---|---|---|
| Tube Material | Medical grade, transparent PET / Glass | ISO 6710, CE | PET |
| Anticoagulant | EDTA Disodium (EDTA-2Na) or EDTA Tripotassium (EDTA-3K) | WHO/CLSI | EDTA-2Na |
| Concentration | 1.5–2.2 mg EDTA/mL whole blood | CLSI H3-A6 | 1.8 mg/mL |
| Tube Volume | 0.5 ml–10 ml (most commonly 2/3/4/5 ml) | ISO 6710 | 2ml, 3ml, 4ml, 5ml |
| Cap Color | Purple / Lavender | CLSI H1-A5 | Purple |
| Sterilization | Gamma irradiation or EO sterilized | USP/Ph.Eur | Gamma irradiation |
| Expiration | 18–24 months (sealed) | ISO 6710 | 24 months |
| Lot-to-lot Consistency | CV < 1.5% | In-house/ISO | <1.0% |
Applications & Use Cases
- Hematology: Routine CBC (Complete Blood Count) and differential blood cell analysis
- Molecular Diagnostics: DNA/RNA extraction, PCR, Next Generation Sequencing sample collection
- Immunology: Flow cytometry, immunophenotyping of blood cells
- Veterinary Medicine: Animal clinical diagnostics
- Epidemiological Studies: Population health sample collection and biobank storage
- Pediatric Capillary Sampling: Micro-EDTA tubes for infants or patients with difficult vein access
The sodium edta tube is essential for safeguarding specimen integrity, preventing coagulation, and enabling accurate downstream analysis in varied real-world clinical settings.
Expert FAQ: sodium edta tube & Related Terminology
- 1. What material is commonly used for sodium edta tube production?
- PET (Polyethylene terephthalate) and borosilicate glass are most common. PET offers crack resistance and transparency, while glass is preferred for niche high purity applications.
- 2. What are the standard tube volumes available?
- Standard volumes include 2ml, 3ml, 4ml, and 5ml, with custom options up to 10ml; microtubes (200–500μL) serve pediatric/low-volume needs.
- 3. What installation or usage standards govern edta vacuum tube in clinics?
- International standards ISO 6710 and CLSI H3-A6 state that tubes must be sterile, labeled, traceable, and compatible with global phlebotomy devices/vacutainers.
- 4. Is there a difference between EDTA-2Na and EDTA-3K anticoagulant?
- Yes; both prevent blood coagulation, but EDTA-2Na is favored for molecular genetics due to lower interference with ions. EDTA-3K is more common in daily hematology.
- 5. How is sterility ensured in vacuum tube edta?
- Through EO (ethylene oxide) or gamma irradiation sterilization, with validation by endotoxin and microbial tests as per USP/Ph.Eur.
- 6. What is the clinical significance of cap color (purple/lavender)?
- Universal “lavender” cap signifies ethylenediaminetetraacetic acid anticoagulant, reducing risk of pre-analytical mix-up. [See more]
- 7. How long can samples in a sodium edta tube be stored before analysis?
- Usually up to 24–48 hours at 2–8°C for cell analysis, DNA/RNA stability may persist for several days depending on additive quality.
EEAT, Authority & References
Professionalism & Trustworthiness: All clinical in-vitro diagnostic consumables provided by Shijiazhuang BDT Heating Pad Co.,Ltd. strictly adhere to ISO, CE, and GMP manufacturing protocols. Our R&D team actively participates in global industry forums and journal publishers such as Annals of Clinical Laboratory Science and regularly reviews updates from LabPulse.com.
- Pre-analytical Variables & Blood Collection Tubes. AACC Clinical Laboratory News
- Recent Innovations in Blood Collection Tubes – Sage Journals
- IFCC: Survey on Anticoagulants and Laboratory Tubes
- NCBI: Pre-analytical Quality in Blood Collection and Tube Additives
- LabPulse: Trends in Blood Collection Tube Technology
For global partnership, OEM/ODM solutions, and detailed specification consultation, contact us directly: bdttonyjiao@hotmail.com | Phone/WhatsApp: +86 150 8111 5902 | sodium edta tube for more information.