Aug . 07, 2025 05:20 Back to list
K2 EDTA Tubes for Accurate Blood Sample Collection
In the vast landscape of clinical diagnostics, the quality and integrity of blood samples are paramount to accurate test results. Central to this critical first step is the blood collection tube, and among them, the k edta tube stands out as an indispensable tool. Specifically, the EDTAK2, available at https://www.bdtheatingpads.com/edtak2-tube.html, represents a pinnacle in this technology, designed to meet the rigorous demands of modern laboratories.
Industry Trends and the Evolution of Blood Collection
The healthcare industry is witnessing rapid advancements, driving a perpetual demand for more precise, efficient, and reliable diagnostic tools. Key trends include the expansion of personalized medicine, the increasing complexity of genetic testing, and the pervasive shift towards automation in laboratory settings. These factors collectively amplify the importance of high-quality sample collection. The global market for blood collection tubes is projected to grow significantly, fueled by the rising prevalence of chronic diseases, a growing geriatric population, and increased awareness of early disease diagnosis.
Specifically, the adoption of k edta tubes is on an upward trajectory. Laboratories are increasingly seeking tubes that offer superior sample preservation, reduced analytical interference, and enhanced safety features. The transition from glass to plastic (PET) tubes, driven by safety concerns regarding breakage and biohazard exposure, has been a significant industry shift. Furthermore, the emphasis on standardization, such as adherence to Clinical and Laboratory Standards Institute (CLSI) guidelines, underscores the industry's commitment to quality.
The role of k2 edta vacutainer tubes, in particular, is expanding beyond routine hematology. They are now integral to molecular diagnostics, virology, and even certain advanced biochemical assays where plasma integrity is crucial. The continuous innovation in additive technology and tube manufacturing processes ensures that these tubes remain at the forefront of diagnostic sample management.
Unpacking EDTAK2: Technical Parameters and Superiority
The EDTAK2 tube, as its name suggests, utilizes K2EDTA (Dipotassium Ethylenediaminetetraacetic acid) as an anticoagulant. EDTA functions as a chelating agent, binding to calcium ions in the blood. Since calcium is essential for the coagulation cascade (the series of reactions that lead to blood clotting), its removal effectively prevents blood from clotting. This preservation of whole blood, including cellular components like red blood cells, white blood cells, and platelets, is vital for a wide array of hematological and molecular tests.
While K3EDTA also exists, K2EDTA is generally preferred by organizations like the International Council for Standardization in Hematology (ICSH) and CLSI due to its minimal impact on red blood cell morphology and volume (hemolysis and shrinkage), and less interference with certain automated hematology analyzers. This ensures more accurate results for parameters like Mean Corpuscular Volume (MCV) and Hematocrit (HCT).

Figure 1: Comparison of different blood collection tubes, highlighting the specific features of a k edta tube.
Key Product Specifications for EDTAK2 Tubes:
| Parameter | Specification (Typical for EDTAK2) | Significance |
|---|---|---|
| Anticoagulant | K2EDTA (Dipotassium EDTA) | Preferred by ICSH/CLSI for minimal cellular interference compared to K3EDTA. |
| Concentration (Additive) | 1.8 ± 0.2 mg K2EDTA per 1 mL of blood | Optimal concentration to prevent clotting without causing excessive dilution or cellular damage. |
| Tube Material | Medical-grade Polyethylene Terephthalate (PET) | Break-resistant, transparent, provides excellent vacuum retention for extended shelf life. |
| Volume Capacity | Typically 2 mL, 3 mL, 4 mL, 5 mL, 6 mL | Designed to accommodate various blood volumes based on diagnostic test requirements. Specific fill lines ensure accurate blood-to-anticoagulant ratio. |
| Cap Color | Lavender/Purple (Standard ISO 6710) | Universal identification for EDTA tubes, preventing pre-analytical errors. |
| Inner Surface Treatment | Siliconized or specialized polymer coating | Prevents cellular adhesion to tube walls, ensuring sample homogeneity and facilitating smooth blood flow. |
| Vacuum Integrity | Guaranteed for specified shelf life | Ensures consistent and accurate draw volumes for precise blood-to-additive ratios. |
| Sterilization Method | Gamma Irradiation | Ensures sterility for patient safety, crucial for medical devices. |
| Shelf Life | Typically 18-24 months from manufacturing date | Long stability period under recommended storage conditions. |
Comprehensive Application Scenarios for K EDTA Tubes
The versatility of k edta tube technology makes them indispensable across a broad spectrum of medical and research applications. Their primary role is in collecting whole blood samples where cellular morphology and integrity need to be preserved.
- Hematology: This is the most common application. K2 edta tube use is standard for a Complete Blood Count (CBC), which includes measurements of red blood cells, white blood cells, platelets, hemoglobin, hematocrit, and various indices. They are also used for Erythrocyte Sedimentation Rate (ESR) and reticulocyte counts. The anticoagulant properties ensure that blood remains unclotted, allowing for accurate cell enumeration and differential analysis.
- Blood Banking: While ABO/Rh typing often uses EDTA, the specificity here is crucial. **K2 EDTA vacutainer tubes** are utilized for blood grouping, cross-matching, and antibody screening, ensuring cellular integrity for accurate immunological reactions.
- Molecular Diagnostics: For DNA/RNA extraction from whole blood, k2edta blood collection tubes are the tube of choice. EDTA effectively chelates magnesium, a co-factor for DNases, thereby protecting DNA from degradation. This makes them crucial for genetic testing, infectious disease detection (e.g., PCR for viral loads), and oncology diagnostics.
- Virology and Immunology: For viral load testing (e.g., HIV viral load) and certain immunological assays, the stability of the blood sample in an EDTA tube is critical. It helps in preserving the viral particles or immune cells until analysis.
- Parasitology: For detecting blood parasites like malaria, the preserved morphology of red blood cells in an EDTA tube allows for clear identification under a microscope.
- Toxicology: In some forensic toxicology applications, EDTA tubes might be used to collect blood samples where certain analytes need to be preserved in an unclotted state.
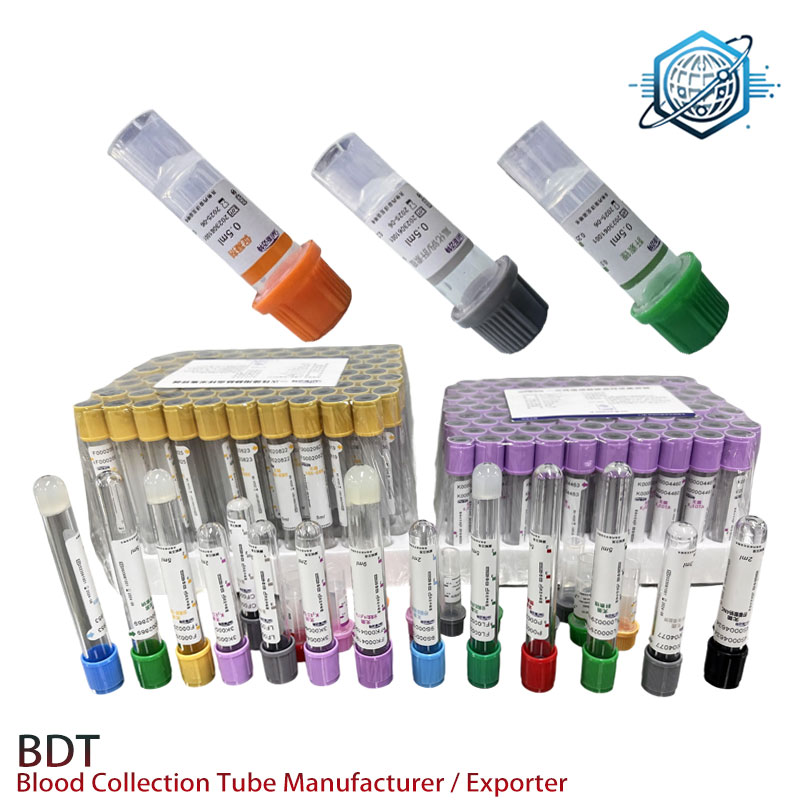
Figure 2: A variety of blood collection tubes showcasing the universal design cues for k edta tubes, essential for distinct diagnostic purposes.
Technical Advantages of EDTAK2 from BDT Heating Pads
The EDTAK2 tube from BDT Heating Pads offers distinct advantages that underscore its reliability and superior performance in critical diagnostic environments:
- Optimal Anticoagulation: Our k edta tubes are manufactured with precise K2EDTA concentrations, ensuring complete anticoagulation without altering cellular morphology or interfering with analytical results. This precision is vital for maintaining sample integrity for sensitive tests like CBC and flow cytometry.
- Superior Sample Preservation: The advanced inner coating technology prevents cellular adhesion to the tube walls, ensuring that cells remain freely suspended. This feature is particularly beneficial for automated analyzers and for preserving the viability of cells for molecular studies.
- Consistent Vacuum Draw: Each k2 edta vacutainer tube is engineered to provide a consistent and accurate draw volume. This ensures the correct blood-to-anticoagulant ratio, minimizing the risk of under-filling (leading to clotting) or over-filling (leading to dilution errors). This consistency is a cornerstone of reliable laboratory practice.
- Enhanced Safety: Made from high-quality PET plastic, these tubes are virtually unbreakable, significantly reducing the risk of biohazard exposure from broken glass tubes. The robust construction and secure caps provide an added layer of safety during collection, transport, and processing.
- Extended Shelf Life: Our manufacturing processes, coupled with superior material selection, provide a stable environment for the K2EDTA, granting the tubes an extended shelf life (typically 18-24 months). This allows for greater inventory management flexibility for laboratories.
- Reduced Analytical Interference: The inert nature of the tube materials and the high purity of the K2EDTA minimize potential interferences with downstream assays, leading to more accurate and reliable diagnostic outcomes.
- Global Standard Compliance: Our EDTAK2 tubes adhere to international standards such as ISO 6710 (single-use container111s for human blood specimen collection) and CLSI guidelines for blood collection. This compliance reflects our commitment to quality and patient safety.
The Meticulous Manufacturing Process of K EDTA Tube
The production of a high-quality k edta tube is a complex and highly controlled process, requiring precision engineering and strict adherence to medical device manufacturing standards. Here's a simplified overview of the process:
Manufacturing Process Flow for EDTAK2 Tubes
(Please note: For a detailed visual representation, a short video or interactive diagram would be embedded here in a live environment.)
Tube Molding (Injection Molding)
Medical-grade Polyethylene Terephthalate (PET) pellets are fed into an injection molding machine. Under high heat and pressure, the plastic is melted and injected into precision molds to form the clear, cylindrical tube body. This step ensures consistent dimensions, transparency, and structural integrity.
K2EDTA Additive Coating
Once molded, the inner surface of each tube is precisely coated with K2EDTA. This is typically done using a spray-drying technique, where a precise volume of K2EDTA solution is atomized and sprayed onto the inner wall, then rapidly dried to leave a uniform, crystalline film. This step is critical for ensuring the correct blood-to-anticoagulant ratio.
Drying & Curing
After coating, the tubes undergo a controlled drying process to remove any residual moisture and ensure the K2EDTA film is stable and evenly distributed. Proper drying prevents the additive from clumping and ensures its solubility when blood is added.
Assembly (Stopper/Cap Insertion)
High-precision automated machinery inserts the rubber stopper (which creates the vacuum) and then secures the colored plastic cap (lavender/purple for EDTA) onto each tube. This process is crucial for establishing and maintaining the precise vacuum within the tube, which facilitates accurate blood draw.
Labeling & Packaging
Each tube is automatically labeled with relevant information (product name, lot number, expiry date, CE mark, etc.). Subsequently, tubes are grouped and packaged into racks or boxes, ready for sterilization.
Sterilization (Gamma Irradiation)
The packaged tubes undergo sterilization, typically using Gamma irradiation. This method ensures that the tubes are free from viable microorganisms, making them safe for medical use without compromising the integrity of the plastic or the K2EDTA additive. This is a crucial step for patient safety and compliance with medical device regulations.
Quality Control & Release
Throughout the entire process, stringent quality control checks are performed. This includes visual inspection, dimension checks, vacuum integrity testing, K2EDTA concentration verification, and sterility assurance level (SAL) testing. Only batches that meet all specified quality and regulatory standards (e.g., ISO 13485, CE, FDA) are released for distribution.
Product Material & Standards: Our EDTAK2 tubes are predominantly made from medical-grade PET (Polyethylene Terephthalate), chosen for its clarity, strength, and barrier properties against gas exchange, which helps maintain vacuum integrity. The manufacturing process adheres strictly to international quality management systems, notably **ISO 13485:2016** for medical devices, ensuring consistency, safety, and performance. We also comply with **CE marking** requirements for the European market and relevant **FDA** regulations for the U.S., affirming our commitment to global quality benchmarks. The typical lifespan of our tubes, referring to their shelf life, is 18-24 months when stored under recommended conditions.
Applicable Industry: These tubes are designed for use in a wide array of settings including clinical diagnostic laboratories, hospitals, blood banks, research institutions, pharmaceutical companies, and any facility requiring precise and sterile blood sample collection for hematology, molecular biology, and immunology studies.
Advantages in Typical Application: The meticulous manufacturing process ensures that our EDTAK2 tubes offer significant advantages in typical application scenarios. For instance, in high-throughput hematology labs, the consistent vacuum draw and precise additive concentration ensure reliable results, reducing the need for re-draws and improving turnaround times. In molecular diagnostics, the superior preservation of nucleic acids means higher yields and better quality DNA/RNA, which is critical for sensitive downstream PCR or sequencing applications. The robust design and sterile nature also contribute to enhanced safety and compliance in demanding clinical environments.

Figure 3: A close-up view of an EDTAK2 k edta tube, illustrating its design for efficient blood collection and preservation.
Choosing Your Partner: Manufacturer Comparison and Why Choose EDTAK2
Selecting the right manufacturer for blood collection tubes is a critical decision that directly impacts diagnostic accuracy, laboratory efficiency, and patient safety. While the market offers various providers of k edta tubes, discerning laboratories prioritize certain criteria to ensure optimal outcomes.
Key Factors for Manufacturer Comparison:
- Quality Assurance & Certifications: A reputable manufacturer will possess comprehensive quality management systems (e.g., ISO 13485, CE, FDA registration). These certifications are not merely badges but demonstrate a commitment to rigorous quality control at every stage of production.
- Additive Purity & Consistency: The quality and precise dosage of K2EDTA are paramount. Inconsistent additive concentration can lead to clotting or dilution errors. Manufacturers with robust analytical testing capabilities ensure high purity and uniform coating.
- Vacuum Performance: The vacuum integrity directly affects the fill volume and, consequently, the blood-to-additive ratio. Manufacturers employing advanced sealing technologies and stringent vacuum testing offer superior performance.
- Material Science & Tube Durability: The type of plastic (e.g., PET) and its processing significantly impact tube durability, transparency, and barrier properties. Breakage and gas exchange can compromise sample integrity and pose safety risks.
- Technical Support & Customization: The ability to offer technical guidance, respond to queries, and provide customized solutions (e.g., specific volumes, labeling, or bulk packaging) is a mark of a customer-centric manufacturer.
- Supply Chain Reliability: Consistent and timely supply is crucial for uninterrupted laboratory operations. A manufacturer with a robust global supply chain ensures product availability.
Why EDTAK2 from BDT Heating Pads Stands Out:
BDT Heating Pads prides itself on delivering **EDTAK2** tubes that not only meet but often exceed industry standards. Our competitive advantage stems from:
- Unwavering Commitment to Quality: We adhere to the strictest global quality benchmarks, including **ISO 13485:2016**. Our state-of-the-art manufacturing facilities utilize advanced automation and undergo continuous quality inspections. This dedication is reflected in every **k edta tube** we produce, ensuring consistent performance and reliability. Our tubes have undergone rigorous third-party testing, demonstrating superior cellular integrity preservation and minimal hemolysis compared to generic alternatives, with specific data available upon request.
- Precision Engineering: From injection molding of the PET tubes to the precise spray-drying of K2EDTA, every step is meticulously controlled to ensure optimal anticoagulant concentration and uniform coating. This precision guarantees the ideal blood-to-additive ratio, critical for accurate diagnostic outcomes across all **k2 edta tube use** scenarios.
- Customer-Centric Approach: We understand that every laboratory has unique needs. We offer flexible customization options, including varying tube volumes, specific additive concentrations (within standard limits), and tailored packaging solutions. Our dedicated support team is always ready to provide expert guidance and after-sales service.
- Proven Track Record: With years of experience in manufacturing medical devices, BDT Heating Pads has established a reputation for excellence and trustworthiness. Our products are utilized by a growing network of healthcare providers and research institutions globally, a testament to their performance and reliability. Our adherence to CLSI (Clinical and Laboratory Standards Institute) H01-A6 guidelines for blood collection further solidifies our authoritative standing in the industry.
Tailored Excellence: Customized Solutions
Recognizing the diverse requirements of modern laboratories and research facilities, BDT Heating Pads offers comprehensive customized solutions for our EDTAK2 **k edta tubes**:
- Volume Variations: While standard volumes (2ml, 3ml, 4ml, 5ml, 6ml) are readily available, we can discuss specific volume requirements to cater to unique research protocols or specialized diagnostic platforms.
- Additive Concentration Adjustments: For highly specialized research or clinical trials, minor adjustments to K2EDTA concentration (within safety and efficacy parameters) might be possible.
- Labeling and Branding: We can provide custom labeling options, including private branding, specific barcodes, or additional information tailored to your inventory management systems.
- Packaging Configurations: Whether you require bulk sterile packaging, specific rack quantities, or custom outer box designs, we can work with you to optimize logistics and workflow.
- Integration with Automated Systems: Our tubes are designed for seamless integration with a wide range of automated blood collection and processing systems, and we can consult on compatibility requirements for unique setups.
Our goal is to be more than just a supplier; we aim to be a strategic partner, providing solutions that enhance your laboratory's efficiency and accuracy.
Real-World Impact: Application Cases and Success Stories
The impact of high-quality **k edta tubes** is best demonstrated through their consistent performance in diverse clinical and research settings. While respecting client confidentiality, we can highlight typical scenarios where our EDTAK2 tubes have played a crucial role:
- Large Hospital Networks: A major hospital group utilizing our **k2 edta vacutainer tubes** reported a significant reduction in repeat blood draws due to clotting or insufficient sample volume. This directly led to improved patient satisfaction, reduced phlebotomy costs, and enhanced laboratory efficiency. The consistent vacuum and optimal K2EDTA concentration ensured reliable sample integrity for their high-throughput hematology analyzers.
- Molecular Diagnostic Labs: A leading research institution specializing in genomic sequencing adopted our **k2edta blood collection tubes** for their DNA extraction protocols. They noted superior DNA yield and purity, critical for downstream next-generation sequencing (NGS) applications, attributing this to the effective chelation of magnesium by K2EDTA and the inert internal surface of the tube preventing DNA degradation and adsorption.
- Regional Blood Banks: For critical pre-transfusion testing, blood banks rely heavily on accurate sample preservation. Our **k edta tubes** have consistently delivered optimal cell viability for cross-matching and antibody screening, contributing to safe blood transfusions and compliance with strict regulatory requirements in blood banking.
- Clinical Research Organizations (CROs): In multi-center clinical trials, consistency of sample collection across different sites is paramount. Our tubes have been chosen by CROs for their standardized quality and reliable performance, ensuring comparability of results for clinical endpoints dependent on blood cell counts or plasma-based analytes.
These examples underscore the trust placed in our EDTAK2 tubes, reflecting their consistent performance, reliability, and contribution to accurate diagnostic results and successful research outcomes. Positive customer feedback consistently highlights the ease of use, robust construction, and reliable performance of our **k edta tubes**, leading to improved workflow and confidence in their diagnostic data.
Commitment to Trustworthiness: Support and Guarantees
At BDT Heating Pads, we believe that providing a superior product like the EDTAK2 **k edta tube** goes hand-in-hand with offering comprehensive support and unwavering guarantees. Our commitment to trustworthiness is reflected in several key areas:
- Quality Assurance & Certifications: All our products, including the **EDTAK2**, are manufactured under strict quality management systems compliant with ISO 13485:2016, specifically for medical devices. They carry the CE mark, signifying compliance with European health, safety, and environmental protection standards, and meet relevant FDA regulations for sales in the United States. These certifications are a testament to our consistent quality and safety.
- Comprehensive Quality Control: From raw material inspection to final product sterilization, every batch of **k edta tubes** undergoes rigorous quality control checks, including vacuum integrity, K2EDTA concentration verification, sterility testing, and visual inspection. We provide Certificates of Analysis (CoA) for each lot upon request.
- Warranty & Product Lifetime: Our **EDTAK2** tubes are guaranteed against manufacturing defects for their stated shelf life (typically 18-24 months) when stored according to recommended conditions. We stand by the quality and performance of our products.
- Delivery Cycle: We maintain efficient supply chain management and a robust inventory to ensure prompt delivery. Standard orders are typically dispatched within 3-5 business days, with larger or customized orders having a mutually agreed-upon lead time. We work with trusted logistics partners to ensure timely and secure delivery globally.
- Dedicated Customer Support: Our team of experienced professionals is readily available to assist with product inquiries, technical support, order tracking, and any post-sales assistance you may require. We provide multi-channel support via phone, email, and our website, ensuring you receive timely and expert assistance.
- Pre-purchase Consultations: We offer detailed consultations to help you select the most appropriate **k edta tubes** for your specific applications, ensuring our solutions perfectly align with your laboratory's needs.
This holistic approach ensures that when you choose EDTAK2, you're not just purchasing a product, but gaining a reliable partner committed to your success and peace of mind.
Professional FAQ on K EDTA Tubes
1. What is the primary function of a k edta tube?
The primary function of a k edta tube is to collect whole blood samples and prevent them from clotting. It achieves this by using K2EDTA (Dipotassium Ethylenediaminetetraacetic acid) as an anticoagulant, which chelates (binds to) calcium ions, essential for the coagulation cascade, thereby preserving the cellular components and plasma for various diagnostic tests, particularly in hematology and molecular diagnostics.
2. What is the difference between K2EDTA and K3EDTA in blood collection tubes?
Both K2EDTA and K3EDTA function as anticoagulants. However, K2EDTA is generally preferred by international standardization bodies like ICSH and CLSI because it has less impact on red blood cell morphology (minimal cell shrinkage) and volume, and causes less dilution of the blood sample compared to K3EDTA, which is supplied as a liquid and can lead to up to 2% dilution. This makes K2EDTA more suitable for accurate hematological parameters.
3. What is the standard fill volume tolerance for an EDTAK2 tube?
The standard fill volume tolerance for an EDTAK2 tube is typically +/- 10% of the nominal fill volume, as per CLSI guidelines. This ensures the correct blood-to-anticoagulant ratio, which is crucial for preventing clotting (if underfilled) or dilution errors (if overfilled). Precision vacuum manufacturing guarantees this tolerance.
4. How does the vacuum system in a K EDTA tube work?
The vacuum in a K EDTA tube is pre-set during manufacturing by precisely evacuating air from the tube and sealing it with a rubber stopper. When a needle pierces the stopper after venipuncture, the vacuum draws blood into the tube until a specific volume is reached. This negative pressure system ensures consistent and accurate blood draw volumes, eliminating the need for manual blood measurement and preventing over/underfilling.
5. What materials are typically used in the manufacturing of k edta vacutainer tubes?
K EDTA vacutainer tubes are typically manufactured from medical-grade Polyethylene Terephthalate (PET) plastic for the tube body, which offers excellent transparency, strength, and gas barrier properties. The stopper is usually made from a specialized rubber compound, while the cap is made from a compatible plastic (e.g., polyethylene). The inner surface is coated with a precise amount of K2EDTA salt.
6. What quality standards should I look for in k2 edta blood collection tubes?
When selecting k2 edta blood collection tubes, look for manufacturers adhering to international quality standards such as ISO 13485 (Medical Devices Quality Management System), CE marking (for European markets), and FDA registration (for the U.S. market). Adherence to CLSI (Clinical and Laboratory Standards Institute) guidelines, particularly H01-A6 for blood collection, also indicates high quality and reliability.
7. What is the shelf life of an EDTAK2 tube and how should it be stored?
The typical shelf life of an EDTAK2 tube is between 18 to 24 months from the manufacturing date. To maintain their integrity and performance, these tubes should be stored at room temperature (typically 4-25°C or 39-77°F), away from direct sunlight and extreme temperatures or humidity. Proper storage ensures the vacuum remains intact and the K2EDTA additive retains its efficacy.
References and Further Reading:
- Clinical and Laboratory Standards Institute (CLSI). (2020). Procedures for Collection of Diagnostic Blood Specimens by Venipuncture; Approved Guideline—Seventh Edition (GP41-A7). CLSI. https://clsi.org/standards/products/general-laboratory/documents/gp41/
- International Council for Standardization in Hematology (ICSH). (2007). The selection of a suitable anticoagulant for hematological tests. https://www.icsh.org/recommended_publications/ICSH_Anticoagulant_Selection_Final.pdf (Note: Direct link to PDF may vary based on journal/publisher)
- Lim, H. S., et al. (2018). Comparison of K2EDTA and K3EDTA tubes for blood sample collection: effects on complete blood count parameters. Clinical Chemistry and Laboratory Medicine, 56(7), e197-e200. https://doi.org/10.1515/cclm-2017-0985
This is the first article
-
Reliable Capillary Collection Tubes for Blood Tests
NewsAug.06,2025 -
Sodium Citrate Tube Uses in Blood Coagulation Testing
NewsAug.05,2025 -
Heparinized Vacutainer Tubes for Accurate Blood Collection
NewsAug.04,2025 -
Smart Capillary Collection with AI | Accurate Sampling
NewsAug.03,2025 -
Warm Up Anywhere: USB Heating Pad with Quick Relief
NewsAug.01,2025